Histopathology Laboratory Services
...our specialist scientific and technical services
WE HAVE MOVED! Temporary moves as part of planned refurbishment.
Cellular Pathology (Cell Path) has temporarily moved from the Royal Victoria Infirmary’s New Victoria Wing to other sites on the shared Newcastle Hospitals / Newcastle University campus.
This move will enable refurbishment to take place, as part of a planned 12-month programme of works. It will also help to prepare the Cell Path site for future service growth.
Currently Cell Path Specimen Reception is located on Level 2 of the Sir James Spence (SJS) Institute with laboratory areas located on Level 5 of SJS and at the Wolfson Building.
Cell Path is planning for this relocation to have minimal clinical impact.
The decant period is expected to last between 9 and 12 months in total and will be followed by a return move back to New Victoria Wing.
Cell Path’s general contact phone number, email address, and mailing address has not changed and if you have any questions about the move the team can be contacted as follows:
Phone: 0191 282 4445
Email: tnu-tr.CellularPathologySecretaries@nhs.net
Mailing address: Level 3, New Victoria Wing, Royal Victoria Infirmary, Queen Victoria Road, Newcastle upon-Tyne NE1 4LP
- Immunocytochemistry
- Immunofluorescence
- Electron Microscopy
- Neuropathology
- Medulloblastoma
- Muscle biopsies
- Nerve biopsies
- Mohs Micrographic Surgery
- Research support
Immunocytochemistry (ICC) is a specialised section of histopathology and the technique involves the identification of specific targets (antigens) within cells and tissues. This involves the use of antibodies that are directed towards the specific targets of interest. The resulting antigen/antibody reaction is then visualised by tagging with a permanent label (chromogen). The presence of the antigen of interest can then be seen by microscopy.
Substances that can be identified by these techniques include micro-organisms, hormones, specific cell types, cellular components, enzymes and tumour markers.
These techniques allow a much greater range of targets to be identified in tissue sections and are of great value in the classification and understanding of disease processes. Examples of the diagnostic applications include the classification of lymphomas, prostatic cancers, skin cancers, hormone status of breast cancers and identification of soft tissue tumours. There is an increasing reliance on ICC as a diagnostic tool which has required a high degree of automation of techniques.
The application of antibodies labelled with a fluorescent tag is a procedure that is performed on fresh frozen skin, oral and renal biopsies. This specialist technique allows for the visualisation of immunoglobulins and complement fractions within the tissues. This technique must be carried out on fresh frozen biopsies because routine fixation affects their demonstration in paraffin sections. The fluorescence is then visualised using a microscope with an ultra-violet light source.
Another technique that is performed on fresh frozen biopsies is the acetylcholine esterase technique. This uses enzyme histochemistry to demonstrate the presence of ganglion cells in rectal biopsies and is employed in the diagnosis of Hirschsprungs disease.
In-situ hybridisation is also performed in the ICC section. This allows the detection of specific RNA sequences within a cell by using specific oligonucleotide probes. Probes currently in use are those for Epstein Barr virus, kappa and lambda light chains, HPV and Her2 gene.
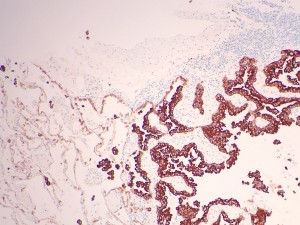
CK5D3
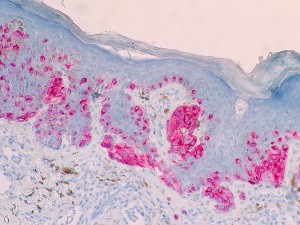
MELAN-A
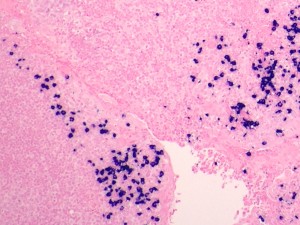
Lambda ISH
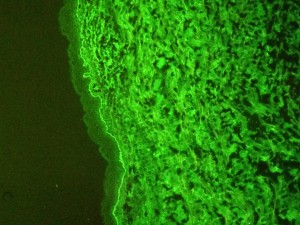
Immunofluorescence C3
Direct immunofluorescence (DIF) is a technique used to diagnose autoimmune bullous diseases in oral mucosa and skin biopsies. In renal tissue it is used to detect and localise immunoglobulins, complement components and fibrin related antigens.
Renal biopsies should be sent fresh to the laboratory in saline-moistened gauze for suitability assessment and sampling. Please ring (0191) 2829133 before sending a renal biopsy.
Oral and skin biopsies should be sent in Michel’s transport medium which is supplied by the laboratory and can be requested by telephoning (0191 282 4270). Michels medium should be stored at room temperature. Each bottle of Michels medium has an expiry date printed on it. Please do not use the samples after this date, simply discard and request fresh solution.
Whilst studies have shown that immunoglobulins are stable in Michels medium for up to 6 months this can result in minor structural changes within the tissue so we advise that samples are not held in Michels medium for any longer than one week. To ensure staining and morphology is maintained we advise you send your samples to us as soon as possible for processing.
Please note:
- Specimens sent in formalin are NOT suitable for IMF studies.
- If there is sufficient specimen it can be bi-sected, one fixed in formalin and one in Michel’s medium.
- Specimens for IMF should be sent to the laboratory immediately.
- Requests for reports on specimens from patients known to have, or suspected of having Transmissible Spongiform Encephalopathy (TSE),Tuberculosis (TB), Human Immunodeficiency Virus (HIV), hepatitis infection, Viral Haemorrhagic Fever (VHF) or any other category 3 or 4 pathogen must be clearly labelled as such and sent fixed and double bagged, marked with a biohazard label on both the request form and specimen container.
See Request Forms & Guidance for:
- Policy for transport of clinical specimens (under ‘Policies’)
- Sample Collection and Acceptance policy (under ‘Policies’)

Transmission electron microscopy may aid in the diagnosis of routine surgical cases. In addition, as a University teaching hospital, research projects are undertaken and many cases are received as referrals.
The ability to resolve ultrastructure contributes in determining the histogenesis of tumours, especially those poorly differentiated tumours in which light microscopical markers are lacking. Seventy five per cent of cases are renal biopsies where visualisation of specific ultrastructural features is often of prognostic value.
Preparation of material is highly skilled requiring dexterity and patience to produce quality ultrasections (100-120nm). The interpretation of digital images is a collaborative effort between the scientific and medical staff.
A specialist area within the Department of Cellular Pathology, it is involved in the histopathological diagnosis of diseases of the central and peripheral nervous system. The service supports the work of the Neurology, Neuro-oncology and Neurosurgery departments within the NUTH Trust, as well as providing a regional specialist referral service. Both surgical and autopsy tissue samples are examined using the extensive resources of the Department.
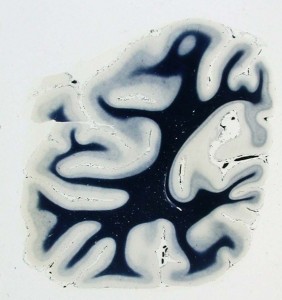
Myelin
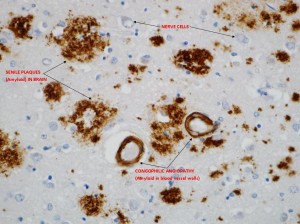
Neuropathology Requests
The standard Histopathology Request Form (Form no. NUTH153) must be completed. Complete patient identification information is essential and full clinical details are also required (refer to Sample collection and acceptance policy – See Request Forms & Guidance for:). In addition, details should be given of any previous biopsies with report numbers where available: previous reports should be found in the patient’s case notes. Please ensure that the Ward number, name of referring Consultant and a contact number are included on the request form.
Request forms with specimens fro m patients known to have, or suspected of having CJD, TB, AIDS, hepatitis B or C or other high-risk infection should identify the biohazard in the section on clinical details. Both request form and specimen container must be marked with a biohazard label. Note that as the processing of CJD material requires special techniques, reports from these cases may take substantially longer than average.
Neuropathology Frozen Sections
Requests for frozen sections should be telephoned to the laboratory, giving at least 30 minutes notice. If the specimen is likely to arrive in the laboratory after 17:00, you must inform the laboratory 0191 282 1482 before 16:00.
Medulloblastoma is a paediatric brain tumour for which Newcastle has been leading with research and clinical trials collaboratively within the UK for a number of years. The majority of medulloblastoma patients in the UK are treated on clinical trials, delivering ‘standard of care’ treatments. Referrals have been made under the clinical trial processes, and we have established an NHS-based national reference service for these purposes, hosted in Newcastle Cellular pathology on behalf of the CCLG and NCRI clinical groups. Referrals and analysis must be completed in real-time to allow treatment selection and trial entry (within 30-40 days post-surgery). And the process has been very successful. Centres are encouraged to continue referring samples to us as standard of care for these patients.
Frozen and FFPE materials can be sent to Newcastle from the treating centre. We then centrally coordinate tissue processing, pathology review (including immunohistochemistry), and molecular testing to produce an integrated report to the trials unit (if trial request) and local treatment centre. In addition to clinical trials, we also accept pathologist to pathologist referrals.
Transport of medulloblastoma samples on dry ice can easily be booked via our specialist courier service provided by CryoPDP. Please contact us to arrange sample transport via Tel. 0191 282 1959.
To ensure that the quality of a muscle biopsy sample is optimal for diagnostic evaluation, it should be handled like a specimen for intra-operative diagnostic evaluation. Thus, the sample has to be sent fresh NOT in formalin or other fluid), covered with a damp gauze, in a universal container immediately after the biopsy procedure to the Cellular Pathology Histopathology reception and the request form should be labelled as “urgent, fresh muscle sample!” Size of the muscle sample should be approximately 5mm x 5mm x 5mm.
The Cellular Pathology reception is located at the New Victoria Wing, Level 3. Preferably the Frozen Section BMS should be contacted (extension: 29133, or from outside the Trust: 0191-282 9133) at the time when the muscle sample is leaving the operation room, so that there is at least a few minutes warning. The fresh muscle sample will be orientated and snap frozen as soon as it is in the laboratory.
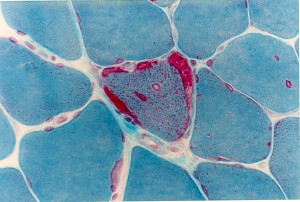
Muscle biopsy-Gomori Trichrome stain showing a ragged red fibre
See Request Forms & Guidance for:
- Muscle Biopsy Referral Form (under Muscle And Nerve Referral Form)
On the day of the Nerve Biopsy the Operating Theatre Staff needs to give a warning to the Frozen Section Staff at Cellular Pathology, RVI 0191 282 9133 (ext. 29133) to ensure they arrive in the theatre in time for the nerve biopsy
15 minutes warning is needed for RVI Operating theatres
See Request Forms & Guidance for:
- Muscle/Nerve Biopsy Referral Form (under Muscle And Nerve Referral Form)
Mohs Micrographic Surgery is a highly successful treatment for skin cancer. The Royal Victoria Infirmary is currently the only hospital in the North East which offers this service. Our Mohs laboratory produce high quality frozen sections for Mohs trained dermatologists to read allowing them to see beneath the visible disease.
The exact location of the cancer cells is pinpointed using a special mapping technique. The cancerous tissue can then be removed in stages until only normal tissue remains. The main advantages of Mohs are its high cure rate and the removal of tissue only where cancer cells are present. This means that the surrounding healthy tissue remains unharmed and reduces the risk of scarring or skin disfigurement. This unique technique is particularly suited for areas where preserving as much normal tissue as possible is extremely important, such as tumours around the eyes, nose, lips and ears.
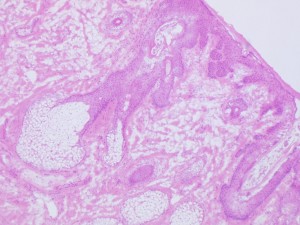
Frozen Section stained in H&E showing a Sebaceous Gland at the Epidermis of a Skin Biopsy
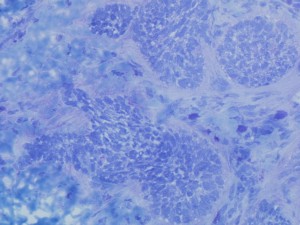
Frozen section of Basal Cell Carcinoma in a skin biopsy, the section has been stained in Toludine Blue





