NEHODS
Our Services
Integrated reporting
Diagnosing haematological malignancies can be a complex process. The WHO classification of haematological malignancy lists over 150 different types of cancers affecting the haematological system. For this reason consultant haematologists and histopathologists use a variety of laboratory techniques to ensure patients are diagnosed and monitored appropriately. Integrating these techniques allows us to make accurate and reliable diagnoses. The diagnosis starts with the clinical history and examination as well as a variety of blood tests. The main tissues and liquids we review are peripheral blood, bone marrow and lymph node but as haematological malignancy can appear in any organ we frequently review specimens from all over the body. In addition to looking at cells and tissues down the microscope we also use more advanced techniques such as immunophenotyping where we analyse markers on the cell surface as well as cytogenetic and molecular testing which can give further information about diagnosis, prognosis and classification. All of these tests are integrated into one report and discussed at multidisciplinary team meetings.
Genetics
Detection of acquired genetic abnormalities in haematological malignancies is essential in the diagnosis, prognosis and monitoring of patients with blood cancer. The genetics team employs numerous techniques to identify clinically significant chromosome and other genetic changes by means of karyotype analysis, FISH and molecular techniques. Some of the tests we use are below:
Karyotyping
G-banded metaphase chromosome analysis (karyotyping) can identify genome-wide structural chromosome abnormalities including balanced translocations, inversion and can identify genomic complexity in living cells actively undergoing mitotic division. Karyotyping is vital in many haematological malignancies especially in myeloid malignancies where karyotype analysis is vital for prognosis and treatment decisions.
FISH
Fluorescence in situ hybridisation (FISH) is generally carried out using commercial probesets on interphase cells from short term cell cultures (e.g. bone marrow or blood) or on FFPE (formalin fixed paraffin embedded) sections or cells released from FFPE blocks or curls. FISH can identify specific gene fusion events such as the Philadelphia chromosome in CML (BCR::ABL1) or deletion of tumour suppressor gene e.g. TP53 in CLL. )
SNP array
Single Nucleotide Polymorphism array testing can identify genome wide copy number changes (gains/losses/gene amplifications) and loss of heterozygosity (LOH).
Molecular testing
We employ a number of methods such as Sanger sequencing, MassARRAY using mass spectrometry technology (MALDI-TOF – Matrix-assisted laser desorption/ionisation – time of flight), targeted PCR fragment analysis, reverse transcriptase PCR, RNA fusion panel, targeted next generation sequencing panels and whole genome sequencing.
Cellular pathology
The lab processes tissue biopsies and specimens from around the region. We specialise in a variety of different pathological techniques. The main tissues we review are bone marrow and lymph node but as haematological malignancy can appear in any organ we frequently review specimens from all over the body. The lab processes the tissues in order to preserve the cellular architecture and stains the sample with a variety of special stains in order to look at a slide under the microscope. Immunocytochemistry (ICC) is a specialised section of histopathology and the technique involves the identification of specific targets (antigens) within cells and tissues. This involves the use of antibodies that are directed towards the specific targets of interest. The resulting antigen/antibody reaction is then visualised by tagging with a permanent label (chromogen). The presence of the antigen of interest can then be seen by microscopy. This allows haematopathologists to examine the types of antigens expressed by malignant cells in order to make a diagnosis. Tissue samples can also be cut for genetic analysis.
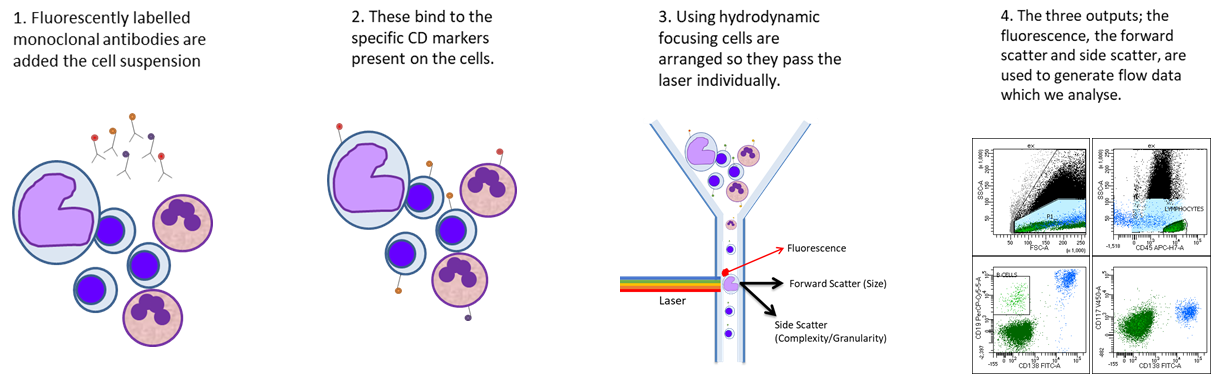
Flow Cytometry
Flow cytometry is a powerful technique which allows us to look at the properties of individual cells and is most commonly used for evaluating peripheral blood, bone marrow, and other body fluids, often detecting tens of thousands of cells per minute. There are three major components to a flow cytometer; the fluidics which transports the sample to the flow cell, the optics which provides the excitation source and collect light signals and the electronics which converts optical signals into electrical signals then into digitized data.
Cells within patient samples are stained with antibodies against ‘CD markers’ on the surface, or inside the cell. These antibodies are conjugated to fluorescent tags which are detected by the flow cytometer. The pattern of CD markers is well documented for both normal cells and malignant populations.
Our test repertoire includes over 80 antibodies, covering around 50 CD markers. We have a range of panels designed to assist in the diagnosis of lymphoproliferative disorders, acute leukaemias, plasma cell dyscrasia, chronic myelomonocytic leukaemia, T cell disorders and a screening tube mainly used in myelodysplastic syndrome investigation. These panels are designed and validated in house by our team of scientists, in accordance with national and international guidance.
Morphology
At NEHODS we review any liquid or tissue sample that may contain a haematological malignancy. Cytological assessment involves reviewing liquid samples such as blood, bone marrow aspirate material, cerebrospinal fluid, ascitic fluid and pleural fluid. After processing and staining, reviewing the liquid using a microscope allows the team to look at the cells that are present and note any morphological abnormalities or abnormal infiltration. Histological assessment involves reviewing solid tissue samples – mainly lymph node and bone marrow trephine but can include review of a sample from any part of the body that may be infiltrated by haematological malignancy such as skin, bowel, spleen and lung. After processing and staining, reviewing the thin sections using a microscope allows the team to look at the structure of the material as well as the cellular content. Morphological examination of liquid and solid material is supplemented special stains and by techniques such as flow cytometry and immunohistochemistry which allows us to determine if an abnormal population of cells is present and the pattern of antigen expression. Bone marrow trephines are reviewed and reported by haematologists and lymph node biopsies are reported by histopathologists.